This study evaluated the effect of low-level laser therapy (LLLT) on the tendency of rat molars to relapse following orthodontic tooth movement (OTM).
Maxillary rat molars were moved mesially for 10 days. Animals were randomly assigned to group I (non-irradiated) or II (irradiation with LLLT). Appliances were removed, and the molars allowed to relapse for 1, 3, 5, 7, 14, or 21 days; rats in group II received LLLT according to a protocol. Bone density of periapical alveolar bone was measured using radiographs and Digora software. Dental supporting structures were examined histologically with haematoxylin and eosin and tartrate-resistant acid phosphatase.
In both groups, first molar relapse was rapid 1 day after the end of active treatment; by 21 days percentage relapse was measured as 86.11 per cent in group I, and 72.22 per cent in group II. Osteoclast number was highest at the end of active OTM, and thereafter successively decreased during the relapse phase in both groups. Decrease in number, and redistribution of osteoclasts occurred more rapidly in the non-irradiated than the LLLT group. Whilst molar relapse was generally less and osteoclast numbers generally higher in group II compared to group I, the differences were not significant. There was no significant difference in bone density between the two groups.
These results indicate that LLLT may reduce the relapse tendency, possibly due in part to bone formation in previous tension areas, and to redistribution of osteoclasts following removal of orthodontic force. The role of LLLT in the prevention of orthodontic relapse requires further study.
Introduction
The biological mechanism of relapse of orthodontically moved teeth appears to be similar to that of orthodontic tooth movement (OTM), and relapse will occur rapidly in the absence of sufficient retention (1, 2). In rats, the mean relapse 1 day after removal of orthodontic appliances ranged from 62.5 per cent to 73.3 per cent, with the rate of relapse decreasing gradually over time (1, 3, 4). In humans, Edman Tynelius et al. (5) stated that the major part of relapse took place during the first year of retention, whilst Kuijpers–Jagtman (6) reported that almost 50 per cent of the relapse occurred within the first 2 years of retention.
This tendency toward rapid relapse has generated the interest to develop methods to reduce or prevent this undesirable change. Various systemically and locally administrated pharmacologic agents have been reported to reduce the amount of relapse in animal models, including bisphosphonate (7), osteoprotegerin (8, 9), simvastatin (10), relaxin (11), and bone morphogenetic proteins (12). The mechanisms of action are varied, but relapse is ultimately decreased by modification of the remodelling process of the dental supporting tissues. Any technique which could alter the normal biologic process following relapse could possibly be used as an adjunct to retention. Low-level laser therapy (LLLT) has been used widely in dentistry; it is a non-invasive tool with various reported bio-stimulatory effects and could therefore be utilized to aid retention (13).
Following a literature review of the influence of LLLT on OTM in both animal and human studies, Torri and Weber (14) found that most authors report that LLLT increases the rate of OTM. Similarly, using meta-analysis of randomized control trials of LLLT use on human subjects, Ge et al. (15) concluded that LLLT could accelerate OTM. Previous studies have observed that LLLT may stimulate the velocity of OTM via increased expression of several key molecules such as RANK and RANKL (16, 17), M-CSF and c-fms (18), MMP-9, cathepsin K, and alpha(v) beta(3) integrin (19). As a result of these molecular reactions, the effects of LLLT biostimulation may be increased bone remodelling, with increased collagen synthesis, bone formation and mineralization, cellular proliferation and differentiation, and angiogenesis (13, 17, 20, 21). Despite the common finding of increased OTM, Goulart et al. (22) observed that a high dose of laser irradiation retarded OTM, and it has been suggested that LLLT may inhibit relapse due to accelerated bone regeneration (13, 23). However, Kim et al. (24) concluded that LLLT would only aid retention if a retainer was in place during the irradiation therapy, otherwise rate of relapse would be accelerated.dental laser
This investigation aimed to examine the effect of LLLT on orthodontic relapse tendencies in a rodent model. It was hypothesized that the biostimulatory effects of LLLT on the dental supporting tissues may minimize relapse after OTM.
Animals were killed by intracardiac perfusion with 10 per cent formalin, following isoflurane inhalation anaesthesia. Measurement of tooth movement was performed again in all rats on the day of sacrifice.
Laser exposure
A photon-plus, gallium-aluminium-arsenide (GaAlAs) diode laser device (Rønvig Dental AS, Daugaard, Denmark) was used, providing a continuous wavelength of 830nm and a power output of 75 mW. The laser beam was delivered by a probe (18mm diameter), with spot size 0.13cm2, and intact power density of approximately 550 mW/cm2. The probe was in light contact with the first molar from the occlusal and lingual sides due to accessibility. Each animal received 3 J/session. Exposure time was 17 seconds, providing an energy density of approximately 23 J/cm2. These conditions were determined based on previous experiments which demonstrated accelerated bone remodelling in bone defects in rats following laser irradiation at energy densities of approximately 20–25 J/cm2 (21, 25).
Histological preparation
Preparation was performed as outlined by Franzen et al. (1). Briefly, following perfusion the maxillae were removed, post-fixed in 10 per cent formalin, demineralized in 10 per cent ethylene diamine tetra-acetate, then embedded in paraffin for histological analysis. Parasagittal sections parallel to the long axis of the first molars were cut at 7 µm and mounted on 3-aminopropyltriethoxysilane coated glass slides. The slide displaying the greatest length of the mesiopalatal root and four adjacent slides were alternatively stained with haematoxylin & eosin (H&E) and tartrate resistant acid phosphatase (TRAP) (in total 12 H&E sections and 15 TRAP sections per animal). The TRAP staining procedure followed the protocol outlined by Brudvik and Rygh (26) using 1 per cent aqueous green counterstain.
Histological analysis
Dental supporting structures of the molars were evaluated in the light microscope. Under high magnification (×100) osteoclasts were counted on the most mesial and most distal roots of the first molars. Cells were considered to be osteoclasts if they were TRAP-positive, multinucleated, and were located on the bone surface or residing in Howship’s lacunae. Cell counts for each section were blindly performed by two operators, following inter-operator calibration. The final count was designated to be the mean of these counts.
Bone density—densitometric analysis
Prior to demineralization, standardized radiographs of the right and left maxillary molars of all rats were taken at a focus-film distance of 40cm, with focus perpendicular to the film-object plane, using a Trophy ETX X-ray machine (Trophy Radiologie, Croissy Beaubourg, France), operating at 70kV, 10 mA for 0.6 seconds. The bone density was evaluated at two periapical areas; mesial and distal to the distal root of the first molar. Mean bone density was measured using Digora software, version 1.51 (Soredex Corporation, Tuusula, Finland). A high definition window mode was chosen in order to delineate the outline of the roots of the first molar. Images were analysed and the mean bone density was measured using Hounsfield units (HU).
Statistical analysis
Data are presented as mean values ± SD. Relapse was calculated as a percentage per group. Group values were evaluated by independent or paired t tests, or one-way analysis of variance where appropriate. Results were considered statistically significant at the P < 0.05 level. Statistical analysis was carried out using the Sigmaplot 12 program (Systat Software Inc., San Jose, California, USA).
Results
OTM and relapse
Following 10 days of orthodontic force application, all treated first molars demonstrated measurable mesial tooth movement, whilst no tooth movement of the untreated contralateral first molars was detected. The mean OTM for group I was 0.19±0.10mm, and for group II was 0.15±0.09mm.
In both groups all appliance-treated molars experienced relapse in a distal direction (Figure 3A); relapsing rapidly 1 day after the end of active treatment (group I: 62.5±14.43%; group II: 54.17±10.21%), with a subsequent reduction in relapse rate (µmd−1) (Figure 3B). By 21 days, the first molars in group I had relapsed a mean 86.11±12.73% of their achieved OTM and those in group II had relapsed 72.22±25.46%. Whilst the molars in group II relapsed less than those in group I at each experimental time point, the differences were not significant.
Material and methods
Animals and experimental procedure
A total of 65 male 6-week-old Wistar rats (HanTac:WH, Taconic, Ry, Denmark), body weight 180–200g, were used. The animals were housed in the Laboratory Animal Unit at The Norwegian institute of Public Health according to a protocol approved by the Norwegian Animal Research Authority, in compliance with the Animal Welfare Act. Body weight of each rat was monitored throughout the experimental period; no significant weight loss was recorded. Four animals were excluded from the study due to appliance complications (final number = 61 rats).
The rats were randomly assigned to group I (non-irradiated) or group II (irradiation with LLLT). The maxillary right first molars of all rats were moved mesially for 10 days using a chrome alloy closed coil spring (0.008×0.030, Ormco, California, USA) ligated to the mesial aspect of the first molar and an incisor band (Figure 1). Activation force was approximately 0.5 N, with no reactivation during treatment. Force magnitude was calibrated by a Correx dynamometer (Haag-Streit, Bern, Switzerland). Appliance placement was performed under anaesthesia by intraperitoneal injection of Ketalar 10mg/ml (Pfizer AS, Lysaker, Norway)/Midazolam 5mg/ml (Alpharma, Actavis Norway AS, Skøyen, Norway), at a dose of 100mg/kg body weight/5mg/kg body weight. All other procedures in the investigation were performed under isoflurane inhalation anaesthesia (Forene, Abbot Scandinavia AB, Sweden).
Figure 1.
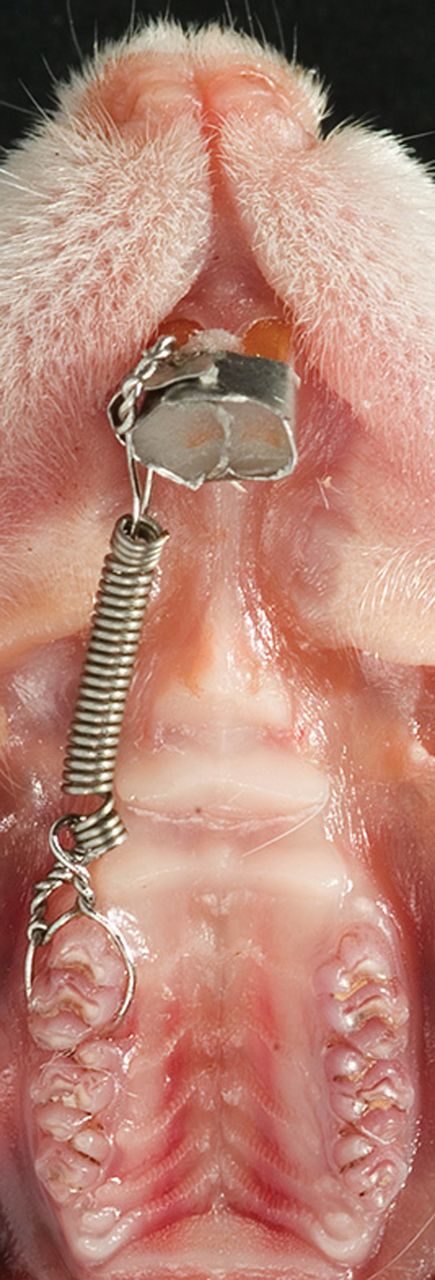
Appliance in situ in rat model. Experimental tooth movement was achieved by mesial movement of the upper right first molar by approximately 0.5 N activation of a closed coil spring.
After 10 days of experimental OTM, appliances were removed, and tooth movement determined using a feeler gauge (Mitutoyo Co.,dental laser, Kawasaki, Japan) with a minimum measurable distance of 0.05mm. Measurements were performed twice by one operator, with no observed variation in the recordings.
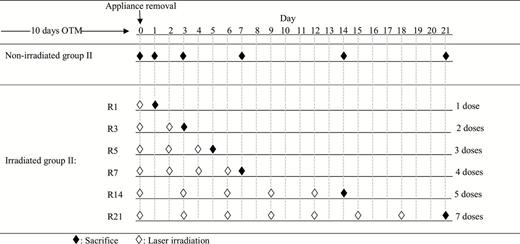
In group I, six rats were sacrificed immediately following appliance removal (I: A0). The remaining animals were killed 1 (I:R1) (n = 4), 3 (I:R3) (n = 4), 5 (I:R5) (n = 6), 7 (I:R7) (n = 5), 14 (I:R14) (n = 5), and 21 (I:R21) (n =5) days following appliance removal. In this group, a split-mouth design was employed, the right half of the maxilla of each animal was experimental and the contralateral sides (left) served as the control group (C).
In group II, the rats were irradiated with LLLT according to varying protocols, and were sacrificed at the same time points as group I (Figure 2). All group II rats received LLLT on the day of appliance removal, and 1 day later six rats were killed (II:R1) (n = 6). Rats killed after 3 (II:R3) (n = 4), 5 (II:R5) (n = 4) and 7 (II:R7) (n = 5) days were irradiated every second day following end of OTM, whilst those killed after 14 (II:R14) (n = 4) and 21 (II:R21) (n = 3) days were irradiated every third day. Thus, the rats in group II, sacrificed at 1, 3, 5, 7, 14, and 21 days received one, two, three, four, five, and seven doses of irradiation, respectively.