Abstract
Objective To determine the safety and efficacy of photodynamic therapy in the treatment of oral leukoplakia with 5-aminolevulinic acid and pulsed dye laser.
Design Nonrandomized, single-arm, single-site phase 1/2 pilot study.
Setting Academic referral center.
Patients A total of 23 patients, aged 37 to 79 years, having a confirmed diagnosis of leukoplakia with or without dysplasia measuring at least 10 mm in diameter.
Interventions Application of 5-aminolevulinic acid to lesions followed by activation with high-power 585-nm pulsed dye laser.
Main Outcome Measures Maximum tolerated dose of laser, postprocedure complications, objective response to treatment, and immunohistochemical changes in treated tissue.
Results No significant adverse events occurred; minor local adverse effects were observed during and following photodynamic therapy in the safety phase of the study. The maximum tolerated dose was 8 J/cm2. Of 17 patients, 7 (41%) had more than 75% regression (significant response) and 9 (53%) had more than 25% regression (partial response), for an overall response rate of 94% at 90 days. This response rate was far higher than the null-hypothesis 20% rate (P < 10−10) and the alternative-hypothesis 50% rate (P = .0001) for which the study was powered. When compared with baseline levels immunohistochemically, p53 expression was increased in 8 of 11 available samples (73%) and Ki-67 expression was decreased in 7 of 12 available samples (58%).
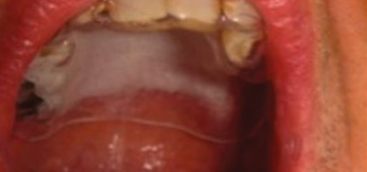
Conclusions Photodynamic therapy with 5-aminolevulinic acid and pulsed dye laser could be used to achieve regression of oral leukoplakia. The treatment is safe and well tolerated. An application time of 1.5 hours and laser radiant exposure of 8 J/cm2 with 1.5-ms pulse time were found to be the optimal settings in this study. The high-power laser used in this study allows completion of laser therapy within 1 to 3 minutes. Further studies are necessary to determine the optimal laser radiant exposure and drug application to maximize the response rate.
Trial Registration clinicaltrials.gov Identifier: NCT00571974
Oral leukoplakia (OL) can be a precursor lesion to oral cavity cancer.1 Currently, the conventional care options for premalignant lesions in the oral cavity and oropharynx are observation, laser ablation,2,3 or aggressive surgical resection. Topical treatments have been attempted to achieve regression of the lesions, including vitamin A (isotretinoin),4 bleomycin sulfate,5 and fenretinide.6 These agents have had varying degrees of success, but none has led to a satisfactory treatment for OL. Surgical resection has been shown1 to be curative, but it is an aggressive intervention associated with significant morbidity when used for large lesions.
Recent clinical pilot studies in Asia7 and Europe8 have shown that photodynamic therapy (PDT) with topical application of 5-aminolevulinic acid (5-ALA) is an effective treatment modality to achieve regression of OL and erythroplakia. 5-Aminolevulinic acid is a metabolite of the heme biosynthesis pathway, which is taken up actively and to a greater extent in tumor cells than in normal cells.9 The agent can be applied topically, and it penetrates well into the skin within 1 to 3 hours.10 The application of 5-ALA to the tissue leads to an accumulation of protoporphyrin IX (PPIX) in the cells. Protoporphyrin IX absorbs light in the visible range (400-700 nm) with distinct 10-nm-wide absorption peaks at wavelengths of 410, 500, 532, 585, and 635 nm. When illuminated with high-energy external light (ie, >4 J/cm2) with a wavelength at either of the absorption peaks, PPIX undergoes a chemical reaction that produces singlet oxygen and other free radicals that are cytotoxic to cells and vessels.
Chen et al7 used a twice-weekly PDT regimen with 5-ALA and a 635-nm light-emitting diode to treat OL in 24 patients. Before administration of PDT, 5-ALA, 20%, was topically applied for 1.5 hours. The optimal application time was determined via fluorescence diagnosis (FD). The maximal fluorescence intensity of the PPIX excited at 410 nm correlates to the maximum PPIX level in the lesional cells. Each lesion was treated repeatedly in 8 sessions separated by 6 to 8 weeks. In each treatment session, the lesions were irradiated with a power density of 100 mW/cm2 for 1000 seconds to a total dose of 100 J/cm2 per treatment. The investigators achieved complete response in 8 patients (33%) and partial response in 16 patients (67%) by the eighth treatment session. Sieroń et al8 treated 24 OL lesions in 12 patients using 6 to 8 treatment sessions separated by 2 weeks with PDT at 635 nm delivered by argon-pumped dye laser with a power density of 150 mW/cm2 for 11 minutes to a total dose of 100 J/cm2. They achieved complete response in 10 patients vs no response in the remaining 2 patients.
In the United States, 5-ALA (Levulan Kerastick; DUSA Pharmaceuticals, Inc, Valhalla, New York) is approved by the Food and Drug Administration to be used in conjunction with a blue light illumination (BLU-U) system for the treatment of actinic keratoses.11 The illumination system consists of a large concave lamp designed to illuminate large areas, such as the face and scalp. The lamp is placed over the patient's head and illuminates 0.01 W/cm2 to the targeted region for 1000 m.

