Abstract
A method for estimating the extent of tooth caries and providing imaging information based on Raman Spectral Imaging is suggested. This non‐destructive optical method is able to characterize and differentiate between normal enamel tooth surface, and initial and advanced tooth caries. Images and corresponding spectra were acquired from various tooth sites, and it was demonstrated that normal, white opaque, brown discoloured, and pitted tooth surfaces all have different distinct spectral features which characterize the different degrees of dental caries. Spectral analysis allows for detection of early changes in the surfaces of carious teeth, and the associated mapping capability allows for morphological characterization. It was found that the emission at 960 cm−1, which corresponds to PO stretching in the hydroxyapatite bond, is the most significant and can be used for diagnosis of caries. The emissions at 1070 cm−1 and at 590 cm−1 can also be applied, but are less accurate. The results suggest that this technique may be further developed and applied for clinical diagnosis of initial and more advanced demineralization processes of the enamel tooth surfaces.
Keywords: Dental enamel, Caries, Mapping, Diagnosis, Laser Raman spectral imaging
Introduction
In a time where a more precise method for dental caries evaluation is needed, optical and laser technology offers a useful tool to detect and characterize physical and chemical changes which occur in calcified tissues.1 Alfano, R. R. and Yao, S. S. 1984. Human teeth, with and without caries, studied by laser scattering, fluorescence, and absorption spectroscopy. IEEE J. Quantum Electron., 20: 1512–1516.
Acidogenic bacteria in the dental plaque, also termed cariogenic bacteria, produce acids, mainly lactic acid, but also acetic and propionic acids, as by‐products of carbohydrate metabolism.2 Loesche, W. J. 1986. Role of streptococcus mutans in human dental decay. Microbiol. Rev., 50: 353–380. [PubMed] Lactic acid dissociates more readily than other acids, producing hydrogen ions that rapidly lower the dental plaque pH.3 Featherstone, J. B.D. and Rodgers, B. E. 1981. The effect of acetic, lactic and other organic acids on the formation of artificial carious lesions. Caries Res., 15: 377–385. As the pH decreases, acid diffuses into the underlying enamel or dentin and dissolves the mineral content,4 Silverstone, L. M. 1973. The structure of carious enamel, including the early lesion. Oral Sci. Rev., 3: 100–160. causing calcium and phosphate ions to diffuse out of the surface, leading to demineralization.5 Featherstone, J. D.B. 1999. Prevention and reversal of dental caries: role of low level fluoride. Comm. Dent. Oral Epidemiol., 27: 31–40. [CrossRef], [PubMed], [Web of Science ®], [CSA]
Historically, the detection of occlusal caries has been performed by using a dental explorer, dental mirror, and light. The tactile sign of resistance when withdrawing the explorer has been considered diagnostic for caries.6 Lussi, A., Imwinkelried, S., Pitts, N. B., Longbottom, C. and Reich, E. 1999. Performance and reproducibility of a laser fluorescence system for detection of occlusal caries in vitro. Caries Res., 33: 261–266. However, this clinical method has a low sensitivity value and, as such, may lead to iatrogenic damage due to incorrect diagnoses.7–9 Ferreira Zndova, A. G., Analoui, M., Beiswanger, B. B., Isaacs, R. L., Kafrawy, A. H., Ecket, G. J. and Stookey, G. K. 1998. An in‐vitro comparison between laser fluorescence and visual examination for detection of demineralization in occlusal pits and fissures. Caries Res., 32: 210–218.
Some studies have investigated fluorescence of teeth by visible light excitation. Alfano and Yao1 Alfano, R. R. and Yao, S. S. 1984. Human teeth, with and without caries, studied by laser scattering, fluorescence, and absorption spectroscopy. IEEE J. Quantum Electron., 20: 1512–1516. 16 Alfano, R. R. and Yao, S. S. 1984. Human teeth with and without caries studied by visible luminescent spectroscopy. J. Dent. Res., 54: 67 found that, when teeth with and without caries were irradiated at 350, 410, and 530 nm, respectively, (using a tungsten light source), emission peaks at 427 nm, 480 nm, and 580 nm appeared. Hibst and Gall17 Hibst, R. and Gall, R. 1998. Development of a diode laser based fluorescence caries detector. Caries Res., 32: 294 used fluorescence spectroscopy with an excitation wavelength of 638 nm or 655 nm and found that fluorescence intensity of caries‐affected teeth can exceed that of healthy teeth by one order of magnitude. This allows for caries to be detected by fluorescence intensity rather than by analyzing spectral differences.
Fisher et al.18 Fisher, L., Feller, L. and Schechter, I. 2002. Tooth‐caries early diagnosis and mapping by fourier transform spectral imaging fluorescence. Instrum. Sci. Technol., 30(2): 225–232. [Taylor & Francis Online], [Web of Science ®] successfully used the Fourier transform spectral imaging fluorescence method to diagnose and map carious lesions. Data collection was performed using micrometer spatial resolution, and the authors concluded that this technique has the potential to become a routine clinical tool in caries diagnosis.
Raman emission is a simple technique which provides information on local chemical bonds or, more precisely, on bond characteristic vibrations. Dental enamel is a composite of ca. 85% minerals, 12% water and 3% protein and lipid (V/V). The mineral component, hydroxyapatite (Ca10(PO4)6(OH)2), has a hexagonal symmetry and the apatite crystalline structure is adaptive to various inclusions; for example, dental apatite contains a substantial amount of carbonate groups, which substitute for the OH− groups (A‐type CO3) or for phosphate tetrahedral (B‐type CO3). Therefore, the general formula of dental hydroxyapatite is [Ca10(PO4)6‐x(OH)2‐y(CO3)x+y].
The sub‐surface carious lesion is less mineralized, more porous, absorbs proteins and bacterial by‐products, and has different optical properties than healthy enamel.10 Featherstone, J. D.B. 2000. Caries detection and prevention with laser energy. Dent. Clin. N. Amer., 44(4): 955–969. It is reasonable to assume that the demineralization process of the enamel should influence the Raman emission spectrum, which is sensitive to both bonds of PO (of the phosphate) and CO (of the carbonate). In this study, the possibility of applying Raman emission bands for estimating the extent of dental caries was examined.
Experimental
Materials
Teeth extracted by dental students at the Medunsa Oral Health Centre, Faculty of Dentistry, Medical University of Southern Africa, South Africa were kept in 5% neutral buffer formalin. Carious teeth from this pool were thoroughly rinsed with water and all organic debris was removed prior to examination.
Experimental Setup‐Raman Spectral Imaging
The experimental setup used for Raman spectral imaging of teeth, is depicted in Figure 1. It consist of a Raman spectrometer from Renishaw, coupled to an imaging microscope (Leica DM‐LM) and a high sensitivity and low noise CCD detector. The latter was thermo‐electrically cooled to –70°C, reaching noise level of 7 e −1 pixel−1 and dark current of 0.0005 e −1 pixel−1 s−1. The microscope was equipped with several objectives (all figures presented here were obtained using the x5 objective). Carious teeth were manipulated using a piezoelectric stage (RGH22) of 0.1 µm resolution.
Figure 1. The experimental setup used for Raman imaging of teeth.
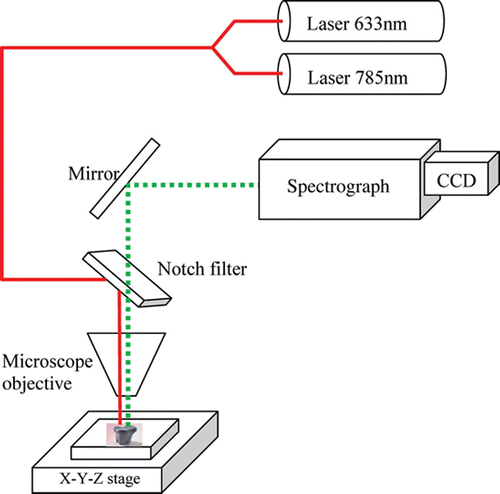
For imaging purposes, a circular area on the sample was illuminated and a tunable filter was used to image the light from a selected Raman band directly onto the detector in a single step. Detailed Raman spectra at points of interest were acquired afterwards, using a grating spectrometer.
Excitation was performed by an air‐cooled HeNe laser, emitting at 632.8 nm in a single mode (TEM000, vertically polarized). The output power was 17 mW. An air‐cooled diode laser, emitting 17 mW output at 785 nm, with true single‐mode operation and a line width of 0.1 cm−1 was also applied.
dental laser tips
Spectral Data Analysis
Due to several geometrical effects, the information obtained from the Raman spectral setup described above is not absolute, but relative. Therefore, data analysis is necessary before evaluation of caries extent is carried out. Spectra were commonly normalized; however, the calculation of peak heights requires some additional consideration. One difficulty is that many of the peaks of interest are superimposed on variable and nonlinear baselines and, in order to overcome this, a polynomial function of the 3rd degree has been fitted to the baseline. For this fitting procedure, all points which do not indicate any specific peak, in the vicinity of the peak of interest (100 cm−1 from each side) were taken into account. The peak height was calculated as the emission intensity above the fitted baseline.
Results and Discussion
The fundamental basis of the detection and quantification of caries by means of non‐invasive diagnostic methods is the exploitation of the physical characteristics of the caries lesion arising from the reduction in the mineral content of the carious enamel and dentin relative to healthy tooth structures.19 Pine, C. M. and Ten Bosh, J. J. 1996. Dynamics of and diagnostic methods for detecting small carious lesions. Caries Res., 30: 381–388. Raman spectra are attributed to the vibronic features of the molecules composing the enamel and dentin structure. The actual composition of organic, inorganic matrix, and proteins are reflected in the Raman intensities at various spectral shifts. Therefore, it is assumed that such data can assist in characterizing degrees of severity of dental caries affecting tooth surfaces, as well as being indicative of the degree of hypomineralization of the tooth structure.
The imaging data of the examined samples is shown in Figure 2. The surfaces seem to be inhomogenous due to the hypomineralization state. The imaging features are not random, but represent the affected enamel and dentin. This conclusion is based on microscope clinical inspection (obtained by moving the stage to various positions). Although the detailed structure depends on the measured location, the general diagnostic characteristics of the image do not change within the same sample.
Figure 2. Sample full view (left) and surface microtopography of dental enamel (right). (a) normal enamel, (b) point of fissure caries, (c) point of light brown moderate caries, (d) point of advanced caries.
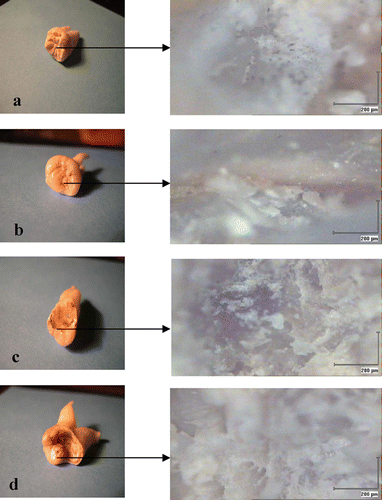
In this study, we refer to four stages of dental caries: normal enamel (no caries at all), fissure carries, moderate caries, and advanced caries. These categories are shown in Figure 2.
The Raman spectra of the samples in Figure 2, at the four representative points, are shown in Figure 3. The Raman spectrometer covers the shift range of 500 to 4,000 cm−1, which is wide enough to provide information on a variety of chemical bonds and vibrational modes.


